Fighting the Flu with a Workplace Prevention Campaign
August 4, 2025
This WorkCare Fact Sheet explains how a workplace flu prevention campaign can help protect your employees and your business during the 2025-26 influenza season.
Implementing a workplace flu prevention plan is important in order to keep employees healthy and productive. Influenza (flu) is a contagious respiratory illness that annually causes work and school absences and productivity disruptions. The flu tends to spread more rapidly during the winter in cold, dry conditions when there is less sunlight and people spend more time indoors.
For the 2025-26 flu season, the U.S. Department of Health and Human Services and the Centers for Disease Control and Prevention (CDC) have endorsed recommendations issued by the nation’s Advisory Committee on Immunization Practices to administer “single-dose formulations of annual influenza vaccines that are free of thimerosal as a preservative for children 18 years or younger, pregnant women, and all adults.”
Benefits of Flu Prevention in the Workplace
Workplace flu prevention campaigns that include access to vaccination help reduce productivity loss and may save lives. The more employees who get vaccinated, the greater the level of protection afforded to everyone, regardless of their vaccination status. This is called herd or group immunity. Flu prevention is particularly important for the protection of infants, frail older adults, and anyone with an underlying condition that increases their risk for pneumonia and other potentially fatal flu-related illnesses.
The flu is estimated to cause at least 17 million days of lost productivity and cost U.S. employers billions of dollars a year. A systematic review of studies on flu-related productivity impacts found that up to 75% of healthy employees missed some work due to laboratory-confirmed influenza, non-confirmed flu-like symptoms, or to take care of an ill household member. Data also show that a majority of employees with symptoms returned to work while possibly contagious. (Refer to Absenteeism and Productivity Loss Due to Influenza or Influenza-like Illness in Adults in Europe and North American, published Dec. 17, 2024, online in Diseases, an MDPI publication.)
The impact of influenza outbreaks on the lives of Americans in terms of related deaths, hospitalizations, the need for outpatient medical care is illustrated in Table 1.
Table 1: CDC disease burden estimates for the 2024-25 flu season
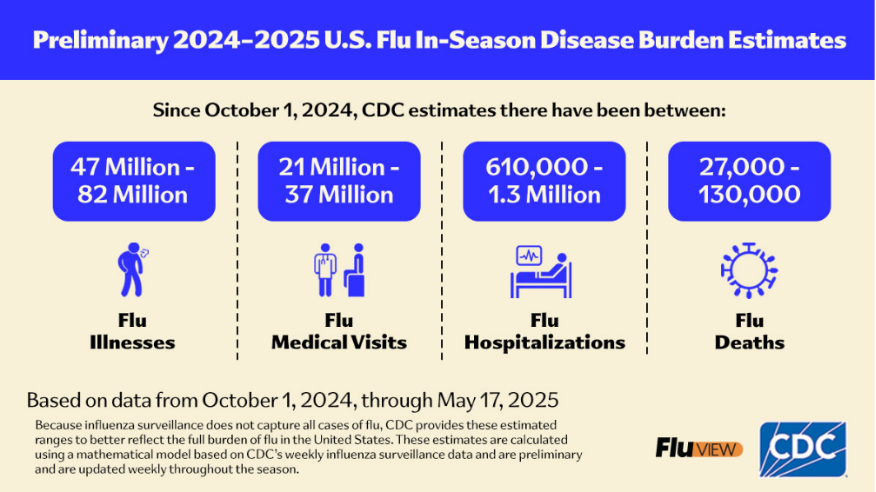
By comparison, there were about 6 million illnesses, 65,000 hospitalizations, and 3,700 deaths reported during the 2023-24 season. Public health officials attribute the 2024-25 season case spike to factors including:
- The virulence of circulating influenza A strains
- Hesitancy and lower vaccination rates
- Declining U.S. population immunity to flu viruses
- Impacts on outpatient care and hospital resources
For the 2024-25 flu season, the CDC estimates that flu vaccination prevented 9.8 million illnesses, 4.8 million medical visits, 120,000 hospitalizations, and 7,900 deaths.
What’s in the 2025-26 Flu Season Vaccine?
The vaccine is formulated to target viral strains that spread in prior northern and southern hemisphere seasons and research on emerging strains. The 2025-26 trivalent (three component) flu vaccine contains two influenza A subtype viruses (H1N1 and updated H3N2) and one influenza type B virus.
Most flu vaccines contain either inactivated (killed) virus or live attenuated (weakened) virus. They are produced using an egg-based manufacturing process. People who are allergic to eggs are advised to ask their medical provider about alternative formulas. For the 2025-2026 flu season, the following are available:
- Injectable inactivated influenza vaccine for children and adults under age 65. There are specific formulations for people older than 65.
- Nasal spray containing attenuated influenza vaccine approved for self-administration and caregiver-administration to children over age 2 and adults up to 49 years old.
- A recombinant, protein-based vaccine that does not contain eggs.
About Thimerosal
This federally approved single-dose, thimerosal-free flu vaccine is contained in a sealed, prefilled syringe. According to the CDC, thimerosal is a compound containing ethylmercury (not to be confused by methylmercury) that has been safely used for decades in multi-dose vaccines to help kill bacteria. While there is no evidence of harm to physical or mental health associated with low doses of thimerosal in vaccine for children or adults, its use has been gradually reduced or eliminated in certain vaccines as a precautionary measure since 1999.
Flu vaccine causes protective antibodies to develop in the body within about two weeks of vaccination. This process may temporarily induce flu-like symptoms, but it does not cause the flu. There is still a chance of getting the flu after vaccination because no vaccine is 100 percent effective. Vaccine effectiveness can vary from year to year, depending on the viruses that predominate. Vaccination has been shown to help reduce illness severity.
Symptoms and Treatment
Most people who get the flu have mild-to-moderate illness and recover on their own within five days to two weeks. Symptoms may include fever and chills, cough, shortness of breath, runny nose or congestion, achiness, headache, and fatigue. Vomiting and diarrhea are more common among children. Some people may develop sinus or ear infections.
Flu remedies include rest, staying well-hydrated, and eating nourishing foods. Over-the-counter medications are available to help relieve symptoms. Antiviral drugs may be prescribed in certain cases to treat symptoms and shorten illness duration. Studies show that antiviral drugs are more effective when they are started within two days of getting sick, although starting them later can still be helpful. Flu antiviral drugs are not used to treat COVID-19.
Contagious Disease Prevention Reminders
Along with vaccination, contagious disease prevention measures employers can recommend to employees include:
- Frequent hand washing with soap and water
- Using hand sanitizer when water is not available
- Covering coughs and sneezes and throwing tissues away
- Disinfecting shared objects and communal areas
- Not touching your nose, mouth, and eyes
- Staying home when ill and avoiding others who are sick
- Wearing a mask, especially when in public places
Legal Considerations
Employers have the right to establish health and safety rules that are job-related and consistent with business necessity. This includes requiring immunizations that protect against the spread of infectious illnesses. Employers who require vaccinations are expected to engage in an interactive process to comply with the Americans with Disabilities Act (ADA), including exemptions for medical necessity or religious beliefs.
State laws require healthcare facilities to ensure consenting employees receive influenza vaccines. In some workplaces, infectious disease management includes mandatory use of personal protective equipment such as gloves, gown, mask, eye protection, face shield and safe injection practices. Surgical masks or respirators may be used to help reduce the spread of disease via airborne or droplet contamination.
How We Can Help You Be Prepared for Flu Season
At WorkCare, we help employers roll out flu prevention campaigns. We support on-site flu shot clinics and assist with referrals to qualified local providers. Our occupational health physicians provide guidance on contagious disease management and return-to-work recommendations after illness.
Contact us to schedule your flu shot clinic.
Flu Vaccine FAQs
When is the best time to get a flu shot?
September and October are considered optimal, but later-season vaccination also help prevent the spread of illness.
What are determining factors for the kind of vaccine a person should get?
Factors that can determine a person’s suitability for vaccination, or vaccination with a particular vaccine, include their age, health status, and allergies.
Will the vaccine give me the flu?
No. Mild, flu-like symptoms may occur while your body develops antibodies that provide protection against viral infections, but you will not acquire a contagious illness.
Why is a flu shot recommended annually?
A person’s immune protection wanes over time, and vaccine composition is adjusted based on viruses that are expected to be in circulation.
Additional Resources
Stay connected and get the latest updates from WorkCare
Let’s Work Together
Ready to take your workforce health and safety to the next level?
Contact us today to learn how WorkCare can partner with you to create a healthier, safer, and more productive workplace.
